COVID-19 / Flu A&B Home Test QUICK REFERENCE INSTRUCTIONS
For in vitro diagnostic use.
For over-the-counter (OTC) use.
For use with anterior nasal swab specimens.
Carefully read all instructions before performing the test. Failure to follow the instructions may result in an incorrect test result. Refer to the Instructions for Use (IFU) for detailed information.


Scan here to visit the product page
An anterior nasal swab sample can be self-collected by individuals aged 14 years or older. Children aged 2-13 years should be tested by an adult.
Warnings and Precautions
Do not use the test if you have had symptoms for more than 4 days or no symptoms at all.
Do not use if any of the test kit contents or packaging is damaged or opened.
When collecting a sample, only use the swab provided in the kit.
All test components are single-use. Do not reuse the test cassette, processing solution, or swab.
Testing should be performed in an area with good lighting.
Do not open the test contents until ready for use. If the test cassette is open for an hour or longer, invalid test results may occur.
Do not use this test if you have been vaccinated with the FluMist/FluMist quadrivalent live intranasal influenza virus vaccine within the last two weeks.
Do not conduct this test if you are prone to nose bleeds or have a nose injury.
Do not use this test if you are using nasal corticosteroids.
Do not use this test if you are using zinc-based throat sprays.
Remove any piercings from your nose before starting the test.
Keep testing kit and kit components away from children and pets before and after use. Avoid contact with your skin, eyes, nose, or mouth. Do not ingest any kit components. The reagent solution contains harmful chemicals (see table below).If the solution contacts your skin, eyes, nose, or mouth, flush with large amounts of water. If irritation persists, seek medical advice.: https://www.poisonhelp.org or 1-800-222-1222 .
| Chemical name | Harms (GHS Code) for each ingredient | Concentration |
|---|---|---|
| ProClin 300 |
Causes skin irritation (H315) Causes eye irritation (H320) |
0.05% |
For the most up-to-date information on COVID-19, please visit: cdc.gov/COVID19.
MATERIALS PROVIDED


Materials required but not provided: Timer or watch.
PREPARING FOR THE TEST
NOTE: Do not open the test materials until ready for use. If the test cassette is open for an hour or longer, invalid test results may occur.
Step 11
Check the expiration date of the test printed on the bottom of the outer box.
Step 22
Ensure all test components are at room temperature (15-30°C/59-86°F) before use.
Step 33

WASH your hands with soap and water for 20 seconds or use hand sanitizer and dry them thoroughly.
Step 44
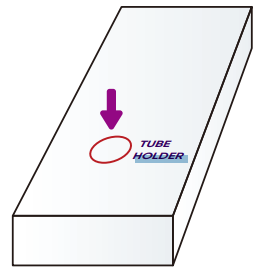
LOCATE the tube holder on the box
(look for the red circle on the kit's box).
Step 55
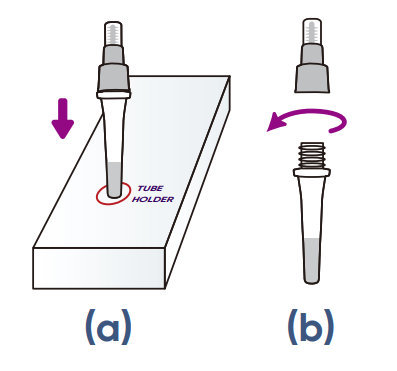
a)
INSERT the buffer tube into the tube holder. Ensure that the buffer tube is stable and upright.
b) REMOVE the large cap from the buffer tube and set it aside for later use.
Step 66
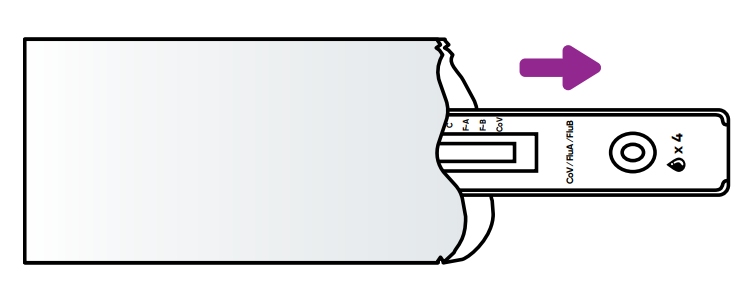
REMOVE test cassette from sealed pouch and lay it on a flat surface.
Sample Collection
Step 77
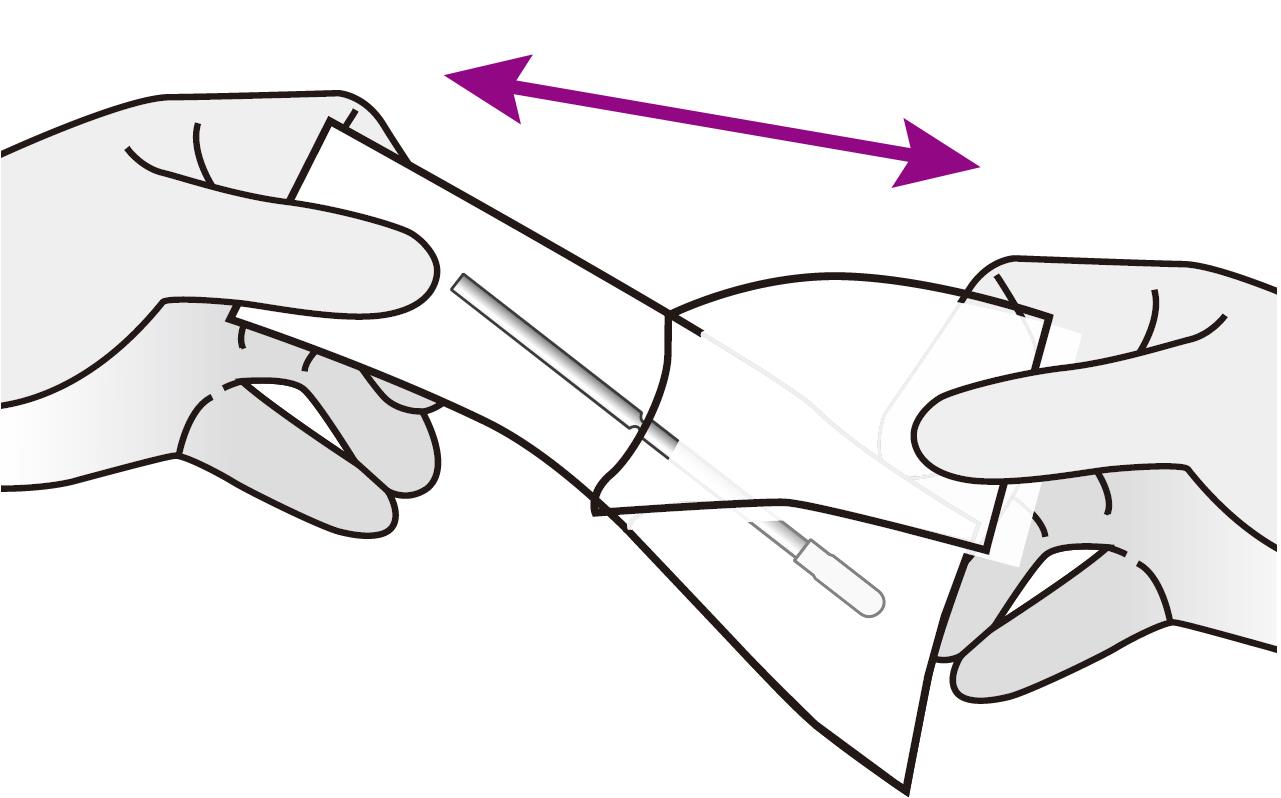
REMOVE the swab from the pouch.
⚠️ Be careful not to touch the swab tip (soft end) with your hand.
Step 8a8a
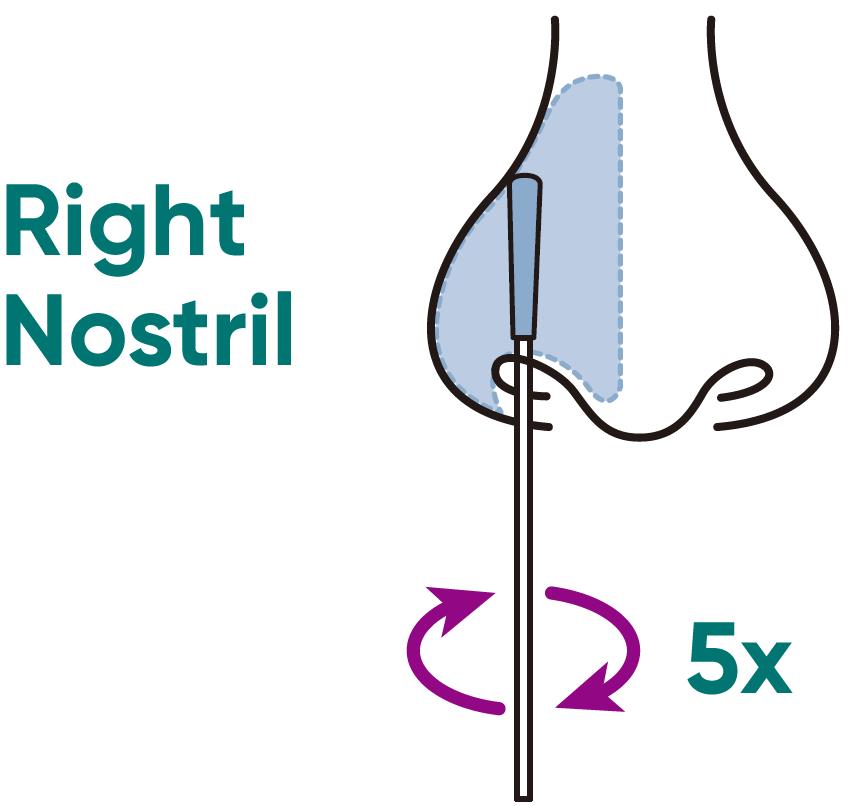
a) CAREFULLY INSERT the swab no more than 3/4 inch (1.5 cm) into the nostril. Slowly rotate the swab at least 5 times against the nostril wall.
⚠️ Do not insert the swab any further if you feel any resistance.
Step 8a8a
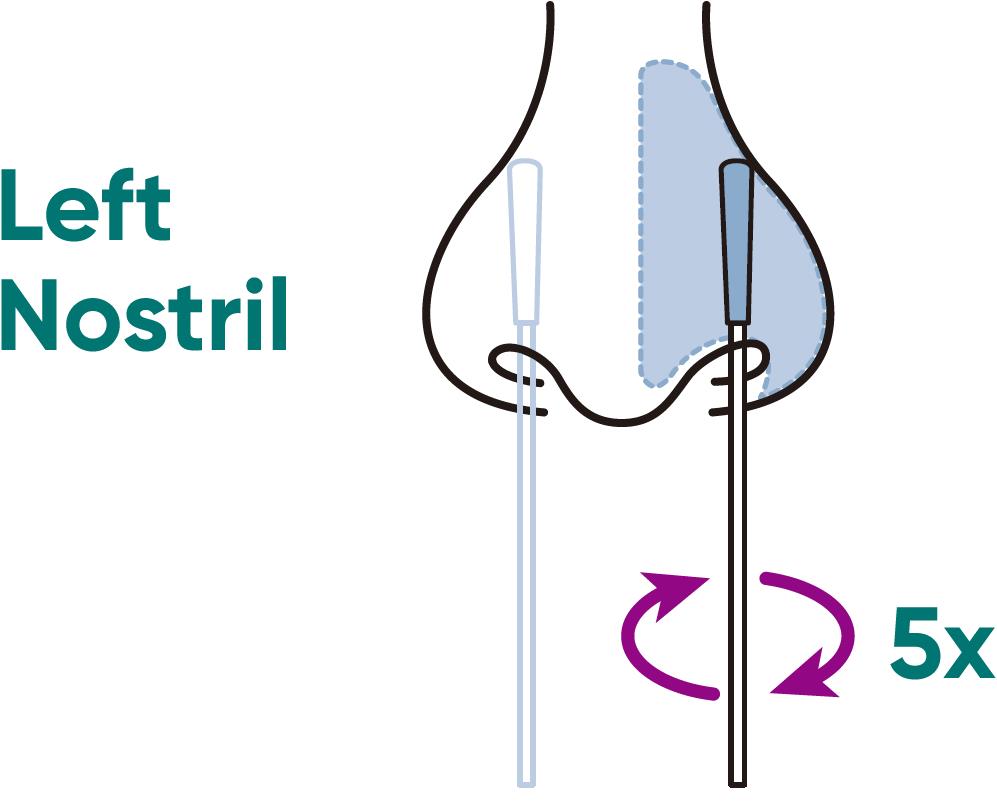
b) REMOVE the swab and repeat in the other nostril using the same swab.
NOTE: If you are swabbing others, please wear a face mask. With children, the maximum depth of insertion into the nostril may be less than ½ to ¾ of an inch, and you may require another adult to hold the child’s head while swabbing. NOTE: Failure to swab properly may cause false negative results.
Running the Test
Step 99
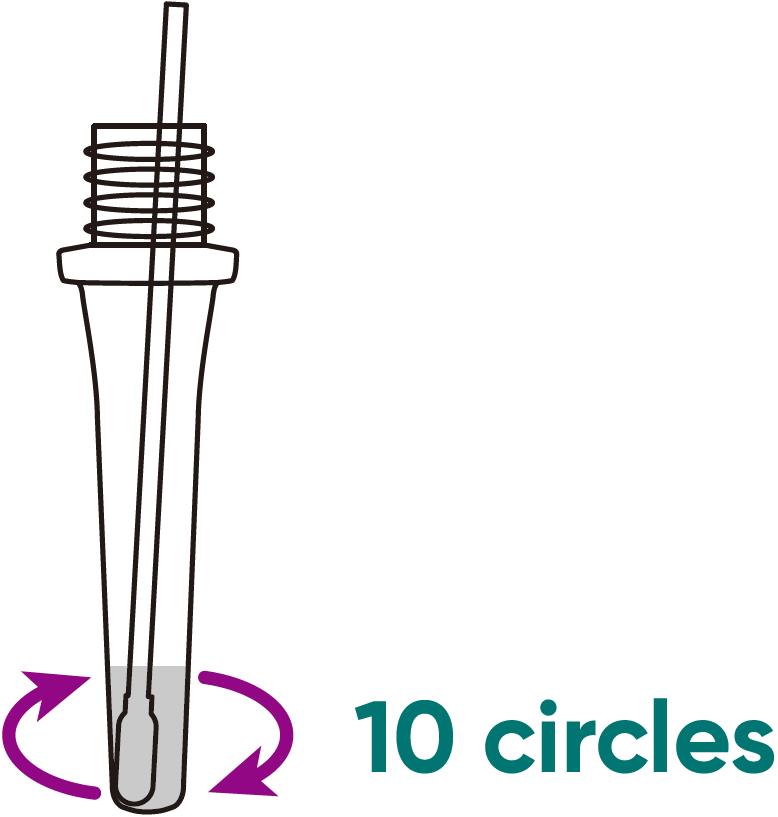
IMMERSE the swab into the buffer tube and SWIRL the swab in the buffer. Ensure the sample is mixed thoroughly by making at least 10 circles.
Step 1010
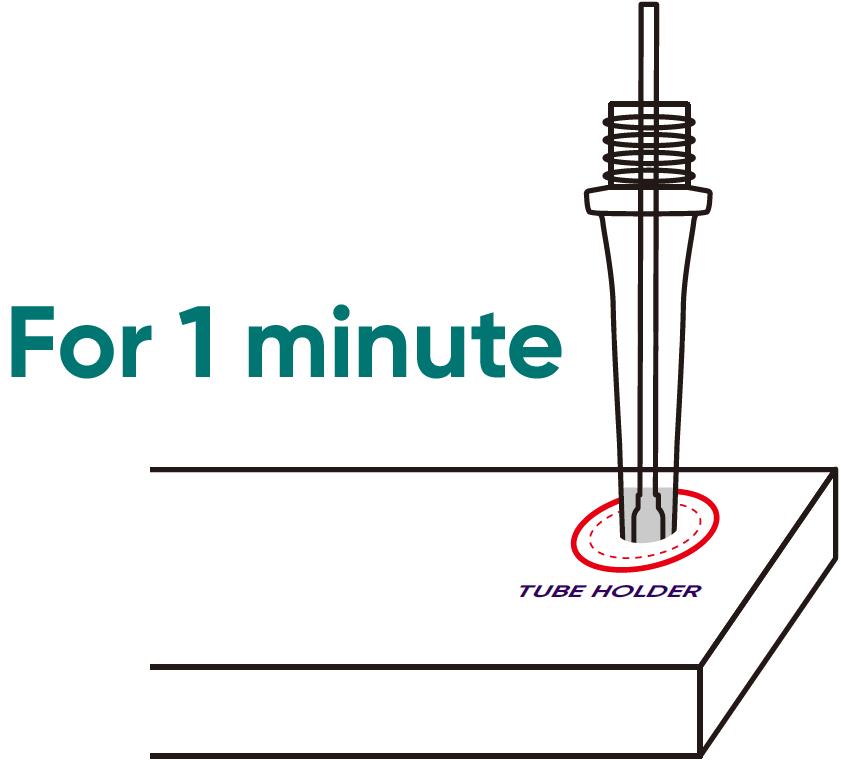
LEAVE the swab in the buffer tube for 1 minute. A timer is recommended for this step.
Step 1111
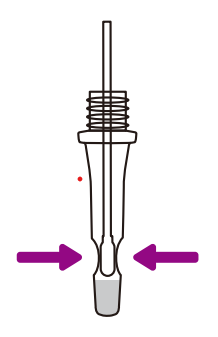
After 1 minute, PINCH the tip
of the swab from the
outside of the tube to
remove any excess liquid
from the swab.
REMOVE and DISCARD the
swab.
Step 1212
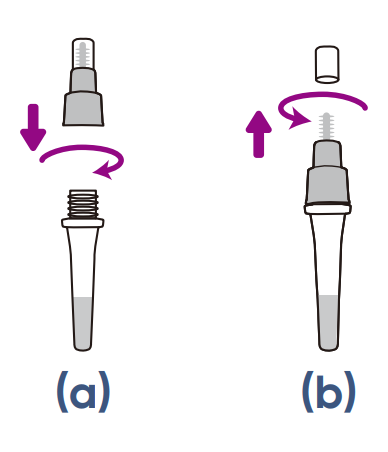
a) HOLD the buffer tube
upright and SCREW the
large cap back onto the
tube. Ensure a tight fit to
prevent leaking.
b) TWIST to open the small
cap at the top of the tube.
Step 1313
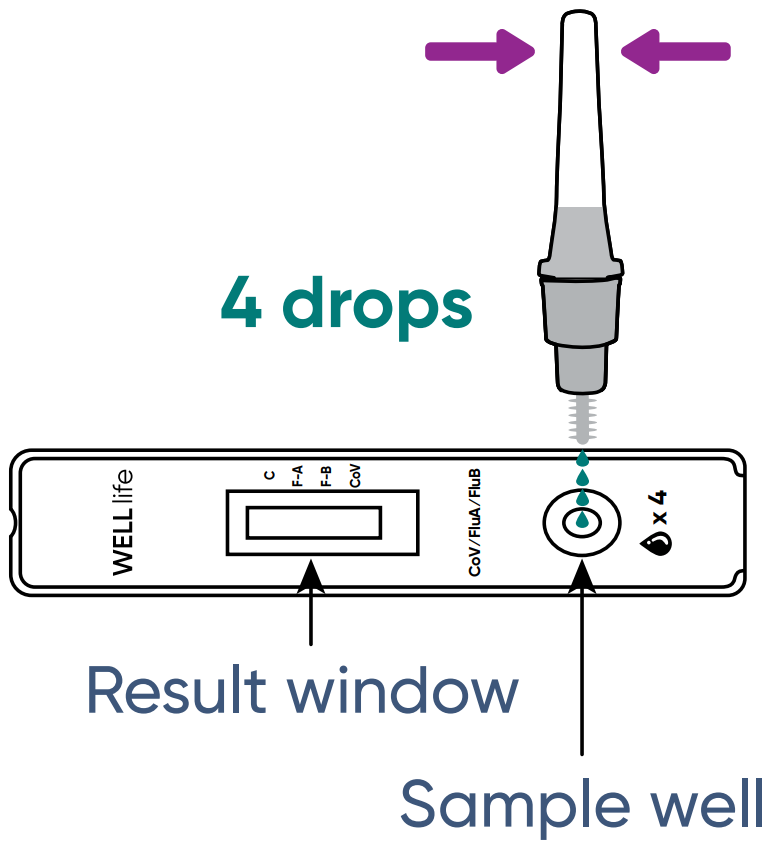
INVERT: the buffer tube and SQUEEZE 4 drops of test sample into the sample well on the test cassette. Then DISCARD the buffer tube.
- NOTE: Incorrect results may be observed if <4 drops of sample are added.
Step 1414

START timer.
Read results at 10 minutes.
INTERPRETING YOUR RESULTS
INVALID RESULT
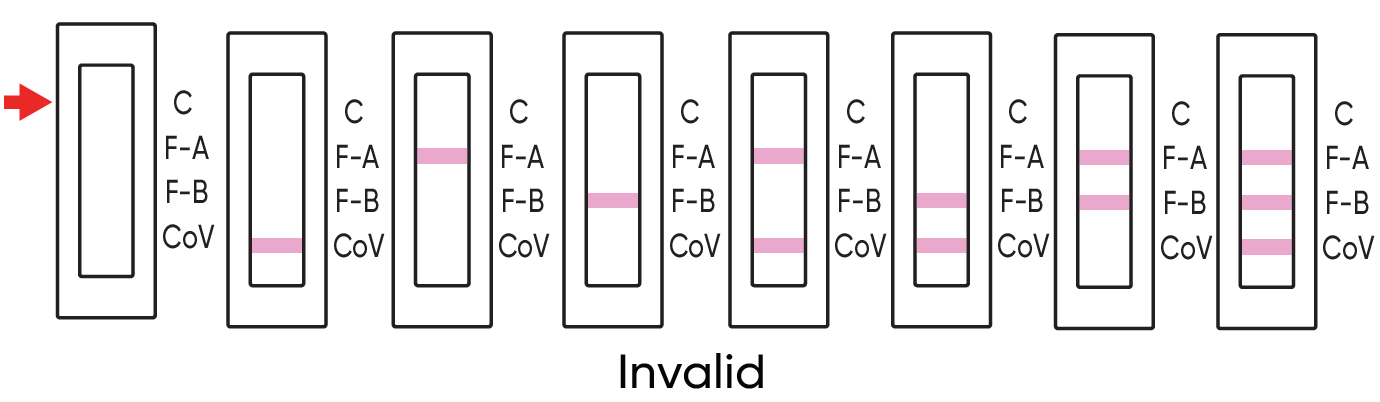
Check to see if a pink to red line is visible at the control line ‘C’ in the results window. If a line is not visible at “C", even if any other line is visible in the results window, the result is considered invalid.
NEGATIVE RESULT
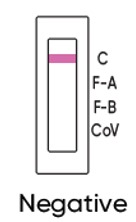
If a control ‘C’ line is visable and you do not see a line at ‘F-A’ , ‘F-B’ or ‘CoV’ , it means the test is negative. The Flu A, Flu B or COVID-19 virus have not been detected.
If respiratory symptoms persist, you should seek follow-up care with your healthcare provider.
POSITIVE RESULT
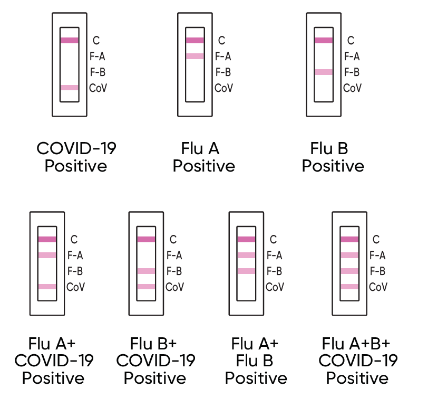
If the control line at 'C' is visible and any other line or multiple lines on ‘F-A’, ‘F-B’ and/or ‘CoV’ are visible, the test is positive for that virus.
NOTE: Any pink to red test line, no matter how faint, should be considered a positive result when the control line is also present.
UNDERSTANDING YOUR RESULTS
RESULTS REPORTING
INTENDED USE
Storage and Stability
- Store the test kit between 36-86°F (2-30°C) in a place out of direct sunlight.
- Reagents and devices must be used at room temperature (59-86°F/15-30°C).
- The unsealed cassette is valid for 1 hour. It is recommended to use the test kit immediately after opening. The expiration date is on the package. Do not use beyond the expiration date.
LIMITATIONS
- The performance of this test was established based on the evaluation of a limited number of clinical specimens collected between December 2023 and March 2024. The clinical performance has not been established for all circulating variants but is anticipated to be reflective of the prevalent variants in circulation at the time and location of the clinical evaluation. Performance at the time of testing may vary depending on the variants circulating, including newly emerging strains of SARS-CoV-2 and their prevalence, which change over time.
- A negative test result may occur if the level of antigen in the sample is below the detection limit of the test or if the sample is collected or handled improperly.
- There is a higher chance of false negative results with antigen tests than with laboratory-based molecular tests due to the sensitivity of the test technology. This means that there is a higher chance this test will give a false negative result in an individual with COVID-19 and influenza as compared to a molecular test, especially in samples with low viral load.
- False positive test results are more likely when the prevalence of SARS-CoV-2, influenza A, and/or influenza B is low in the sample.
- Persons with risk factors for severe disease from respiratory pathogens (e.g. young children, elderly individuals, individuals suffering from chronic lung disease, heart disease, a compromised immune system, diabetes, and other conditions) should contact a healthcare provider; users should also contact a healthcare provider if symptoms persist or worsen.
- This test is read visually. Because test lines can be very faint, users with conditions affecting their vision – such as far-sightedness, glaucoma, or color blindness – are encouraged to seek assistance to interpret results accurately (e.g., reading glasses, additional light source, or another person). This test has not been validated for use by those with color-impaired vision.
- This device is a qualitative test and cannot provide information on the amount of virus present in the specimen.
- This test detects both viable (live) and non-viable influenza A, influenza B, and SARS-CoV-2. Test performance depends on the amount of virus (antigens) in the sample and may or may not correlate with viral culture results performed on the sample.
- Hand soap and hand sanitizers may cause false negative results with this test.
- FluMist / FluMist quadrivalent live intranasal influenza virus vaccine may cause false positive influenza A and B results with this test.
- Zinc-based throat sprays may cause false positive influenza A results with this test.
- Nasal corticosteroids may cause false negative results with this test.
- This test does not distinguish between SARS-CoV and SARS-CoV-2.
FREQUENTLY ASKED QUESTIONS
Potential risks include:
- Possible discomfort during sample collection.
- Possible incorrect test result (see Warnings and Result Interpretation sections for more information).
Potential benefits include:
- The results, along with other information, can help you and your healthcare provider make informed recommendations about your care.
- The results of this test may help limit the spread of COVID-19 and flu to the family of the tested individual and others in your community.
There are different kinds of tests for the viruses that cause COVID-19 and the flu. Molecular tests detect genetic material from the virus. Antigen tests, such as the WELLlife™ COVID-19 / Flu A&B Home Test, detect proteins from the virus. Due to the lower sensitivity of antigen tests, there is a higher chance this test will give you a false negative result when you have COVID-19 than a molecular test would.
A positive result means that it is very likely you have COVID-19 or influenza because proteins from the virus that causes COVID-19 were found in your sample. You should self-isolate from others and contact a healthcare provider for medical advice about your positive result.
A negative test result indicates that antigens from the virus that causes COVID-19 or influenza were not detected in your sample. If you have a negative result, it does not rule out SARS-CoV-2 or influenza infection; you may still be infected and you may still infect others. It is important that you work with your healthcare provider to help you understand the next steps you should take.
For more information on the performance of the test and how the performance may apply to you, please refer to the performance data in the Instructions for Use (IFU), available at: https://wondfousa.com/.
An invalid result means something with the test did not work properly. If the test is invalid, a new swab should be used to collect a new nasal specimen and you should test again with a new test.
Contact Wondfo Product Support at +1 (888) 444-3657 (9:00 a.m. to 5:30 p.m. CDT M-F) or wondfo@wondfousa.com.
IMPORTANT: Do not use this test as the only guide to manage your illness. Consult your healthcare provider if your symptoms persist or become more severe. Individuals should provide all results obtained with this product to their healthcare provider.
If uncertain how to proceed, contact Technical Assistance at +1 (888) 444-3657 (9:00 a.m. to 5:30 p.m. CDT M-F) or wondfo@wondfousa.com.
INDEX OF SYMBOLS

Do not re-use
Use-by date

Keep dry

Batch code

Consult instructions for use
Keep away from sunlight
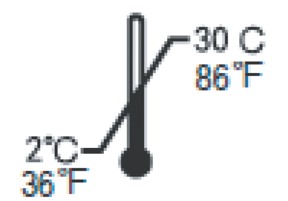
Store at 36~86°F/2~30°C
Manufacturer

Catalogue number
Do not use if package is damaged

In Vitro diagnostic medical device
SUPPORT
If you have questions regarding the use of this product, or if you want to report a problem with the test, please contact Wondfo Product Support at +1 (888) 444-3657 (9:00 a.m. to 5:30 p.m. CDT M-F) or Wondfo USA Co., Ltd. Product Support website: https://wondfousa.com/.

Wondfo USA Co., Ltd.
6720 Cobra Way, San Diego, CA 92121 +1 (630) 468-2199 www.wondfousa.com Made in USA
Rev. B1
Rel.: 2025/04/28
