Discover more from HORMONElife™ by clicking here.
HORMONElife™: Personal Predictions – When it Matters Most
About Us
Wondfo USA
Wondfo USA Co. Ltd. is headquartered in Bolingbrook, IL and is a leading manufacturer of point-of-care tests providing rapid diagnostic and chronic disease management solutions to support the health and well-being of people everywhere. Our mission is to provide solutions that save lives as well as improve quality of life.
Founded in 2009, Wondfo USA has been serving the North American market for over 15 years and we are dedicated to consistently expanding our product portfolio to meet increasing clinical and diagnostic needs.
We manufacture and distribute rapid point-of-care products in 4 major categories including Toxicology Testing, Infectious Disease, Women’s Reproductive Health, and Veterinary Diagnostics.
Wondfo's mission is to bring the benefits of scientific testing to everyone.
Our San Diego research and manufacturing center brings high quality diagnostics to life.
News
Explore how Wondfo is contributing to healthcare innovation
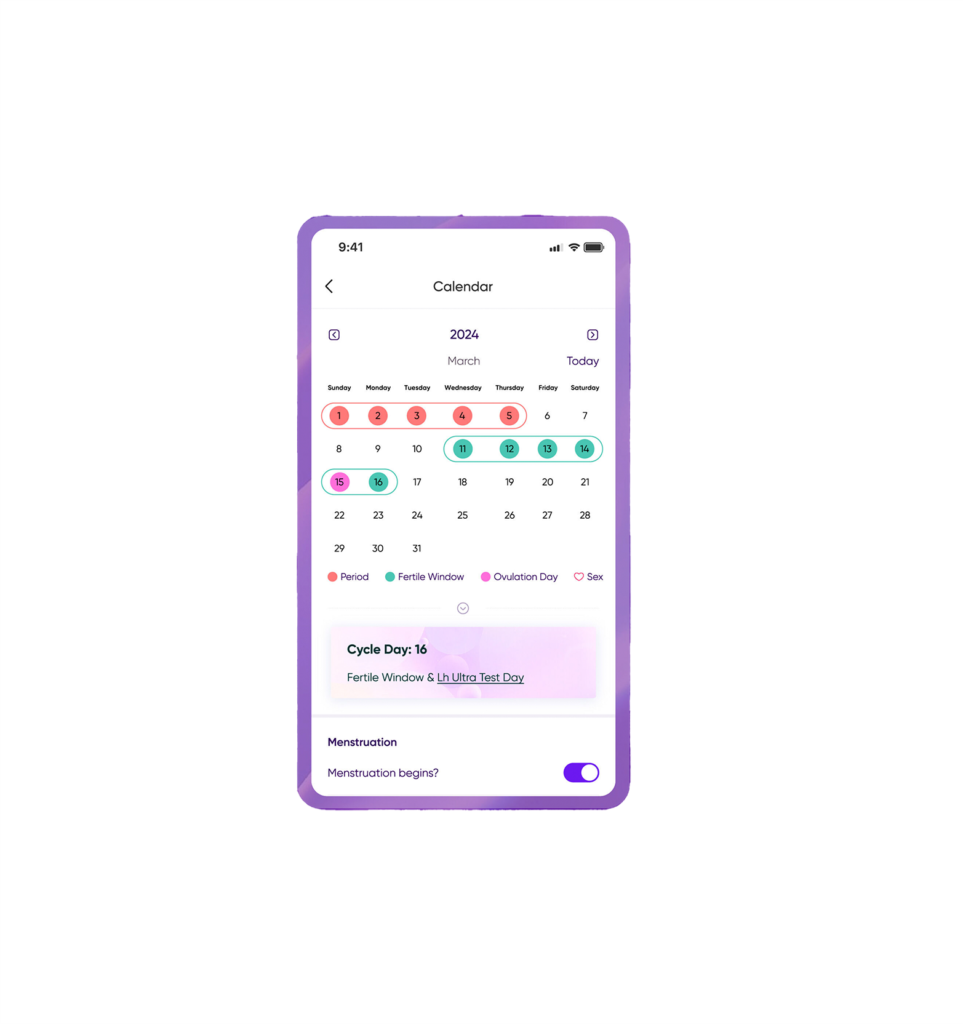
Introducing the HORMONElife App
Introducing the HORMONElife™ App: Your Ultimate Fertility Companion We’re thrilled…
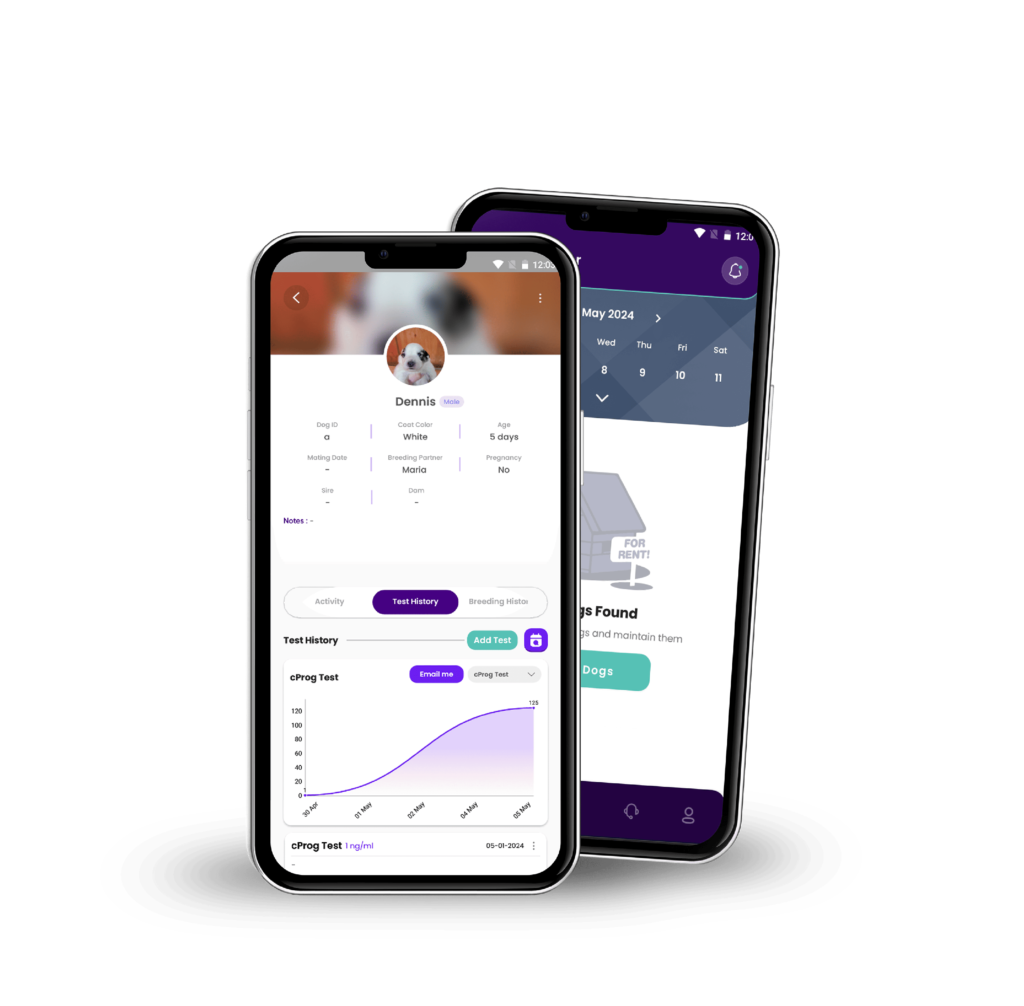
Pup Planner app launched at Wondfo USA!
Discover our new app, designed to help plan and track…
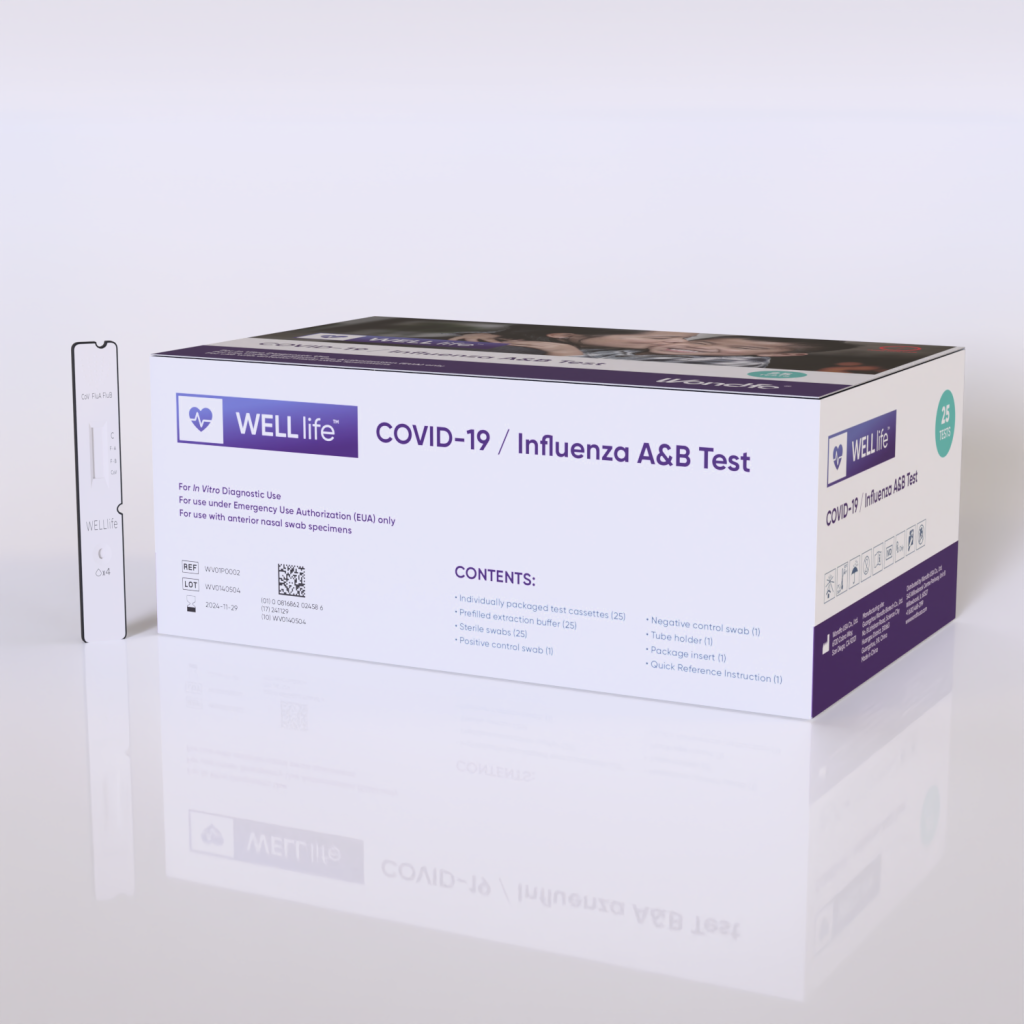
Wondfo’s WELLlife™ COVID-19 / Influenza A&B test receives Emergency Use Authorization (EUA).
The test distinguishes between SARS-CoV-2, Influenza A, and Influenza B with a…
Newsletter
Subscribe for updates.
Subscribe Now
Subscribe for free resources and news updates.